- Thank you received: 0
WHO'S ON MARS? (continued)
Please Log in or Create an account to join the conversation.
<i>Originally posted by Jim</i>
The point I'm making is the nitrogen ion is required to be in place prior to the beginning of life.
Jim
The nitrogen ion, N+3 or N-3, only exists in a plasma at over 2,500 degrees F. It does not exist in the aqueous phase. What does exist in the aqueous phase is NO2- or NO3- or NH4+ and so on. The nitrogen atom ingages in chemical reactions only after its molecular triple bond has been broken and it acquires hydrogen or oxygen attachments. Prior to life, lightning would be the most plausible source of simple nitrogen compounds which can undergo additional reactions. One might postulate the creation of ammonia in a hot vent at the bottom of the ocean, where N2, H2 and iron oxide come together.
Gregg Wilson
Please Log in or Create an account to join the conversation.
- xterrester
-
Topic Author
- Offline
- Elite Member
-
- Thank you received: 0
Please Log in or Create an account to join the conversation.
- xterrester
-
Topic Author
- Offline
- Elite Member
-
- Thank you received: 0
Please Log in or Create an account to join the conversation.
- xterrester
-
Topic Author
- Offline
- Elite Member
-
- Thank you received: 0
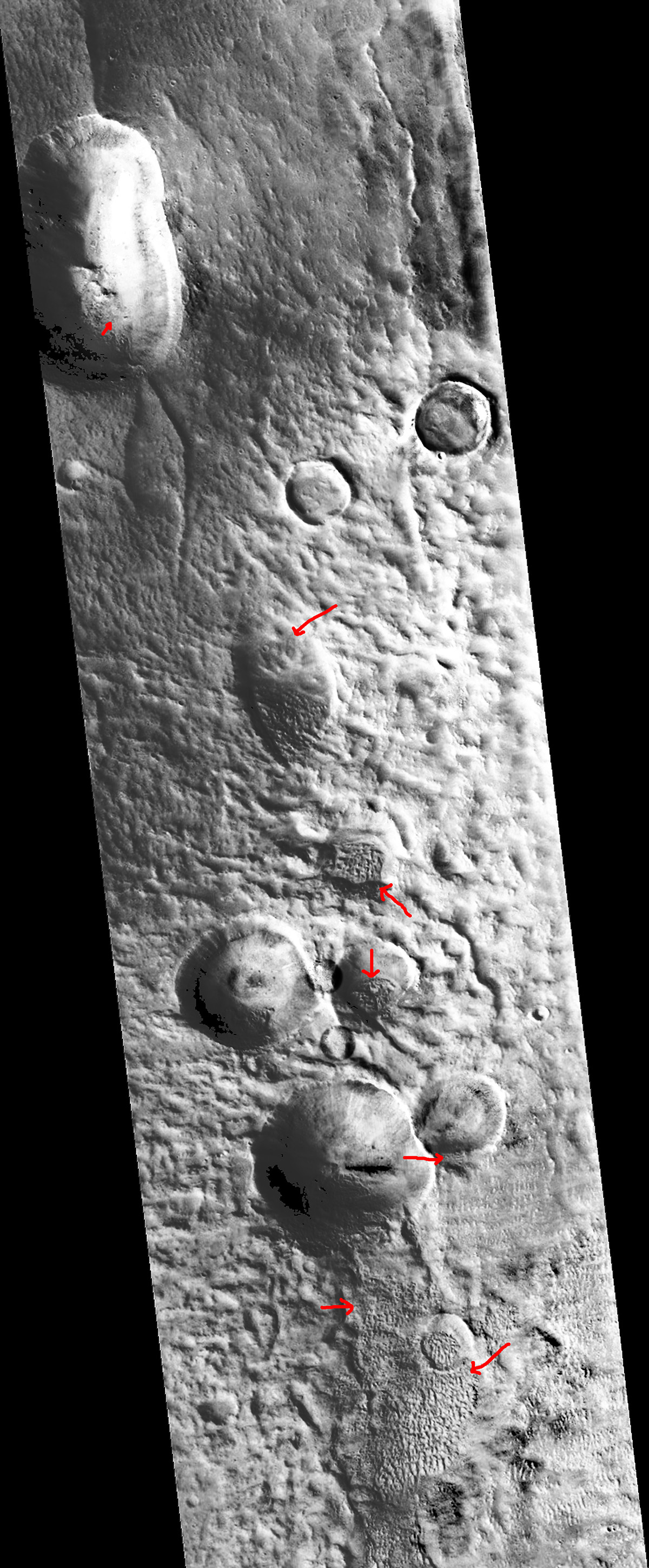
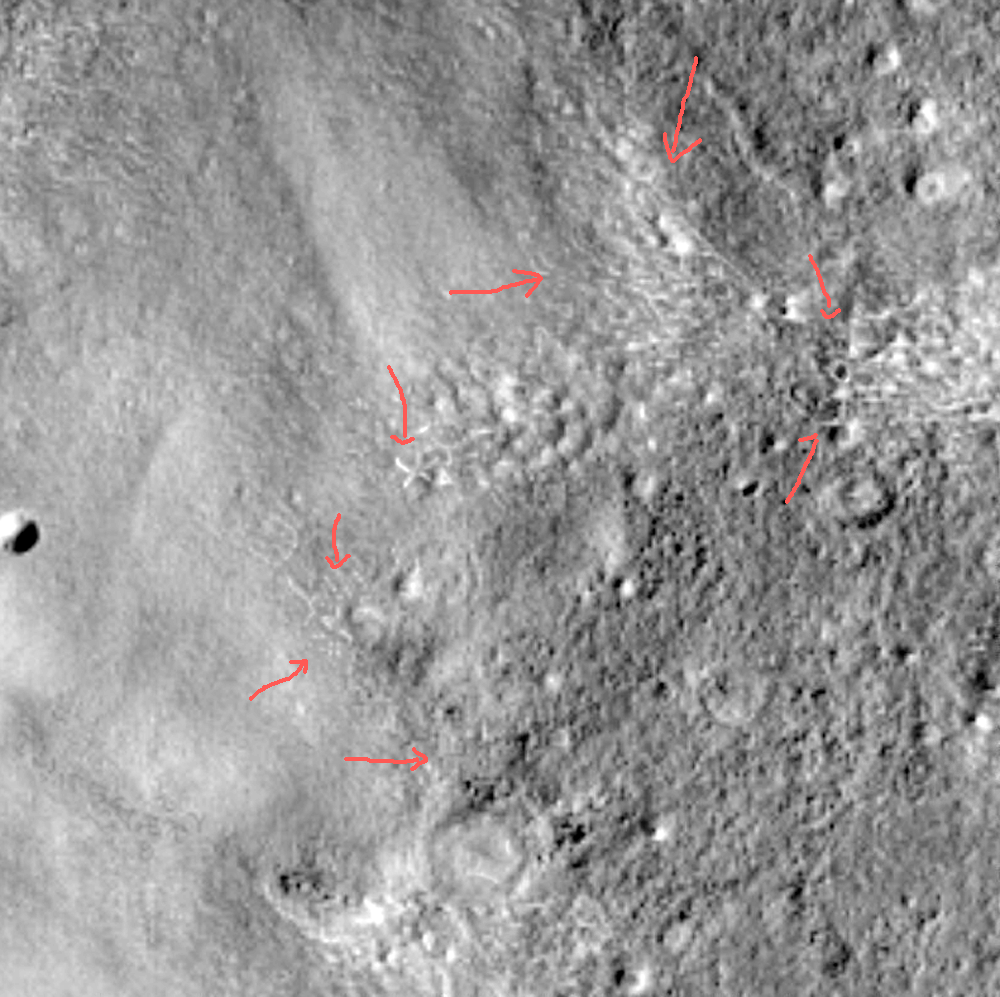
I am amazed by what I see in this image and think it is worth a careful study with a magnifying glass. The red arrows point out just a few of the highlights. In the lower left corner, about three inches up from the bottom edge, a string of markings that resemble alpha-numeric symbols.
Please Log in or Create an account to join the conversation.
- xterrester
-
Topic Author
- Offline
- Elite Member
-
- Thank you received: 0
Please Log in or Create an account to join the conversation.